
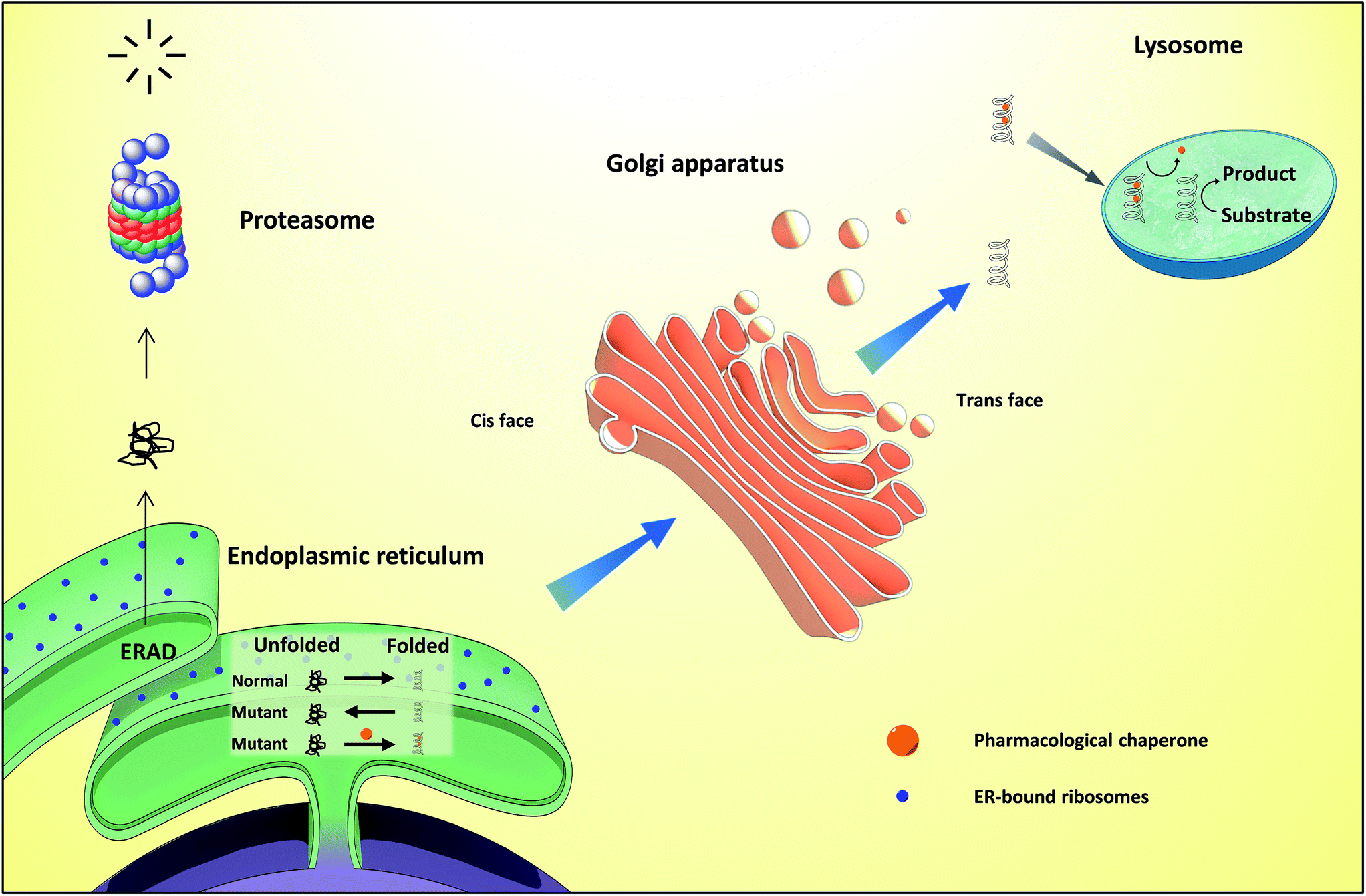

Apart from biology and all its awesomeness, he loves music, animals, and spending time with his family.Dobson, C. He believes that an interdisciplinary approach to science can help us to look at the same question from different perspectives. He wants to understand the role of different chaperones and their effects on the folding-unfolding dynamics of the substrate proteins. He obtained his master’s degree in life sciences with a specialization in plant science, post which he moved to the Structural mechanobiology lab with Dr. Hence, at a single molecular resolution, we observed the chaperone's behavior from a different dimension providing a holistic idea about their diverse nature of action. It was observed that model chaperones after interacting with the focal adhesion protein accelerate their unfolding than its native force range, making it mechanically weak thus, changing the downstream cellular mechanosignalling. We also discovered the role of chaperones in regulating folding unfolding dynamics of model focal adhesion protein to understand their regulatory role in cellular mechanotransduction. We observed that pharmacological chaperones also stabilize both these model proteins substrates containing only alpha helix and containing both alpha helix and beta sheet when they were denatured using mechanical force. We found out that chaperones' ability to withstand deformation was making this difference in behavior between the same types of physiological chaperones.Īfter understanding the mechanical roles of physiological chaperones, we tried to unravel the role of pharmacological chaperones in regulating folding-unfolding dynamics of structurally and biologically different proteins. Not only BiP but also its co-chaperone, ERdj3, was behaving in the same way which was completely opposite to the same class of chaperones like DnaK and DnaJ. These chaperones were previously known to stabilize the unfolded state of protein however, we observed its complete change of behavior when the substrate with which it was interacting was applied force using magnetic tweezers. BiP (a eukaryotic endoplasmic reticulum Hsp70 chaperone) also remains near the Sec61 tunnel towards the ER lumen and works along with ERdj3 (a Hsp40 type co-chaperone). However, there have been reports that say chaperones (bacterial origin) remaining near tunnel proteins actively participate in folding the substrate proteins, when these substrates experience mechanical force. Previous literature on chaperones majorly states their effect in preventing misfolding of proteins by stabilizing their unfolded states and hence guiding the proteins towards achieving their native conformation. Failing to do so might be catastrophic for a cell which might have a domino effect finally resulting in cell death or physiological abnormalities. Chaperones help in giving proper guidance to newly translated polypeptides in a cell and make sure they don't get misfolded. are related to the improper folding of proteins. As physiological abnormalities like Alzheimer's, Parkinson’s, Huntington’s diseases, etc.
Understanding chaperone and their mechanisms has been a frontier area of research in biology for more than half a century. Title: Understanding the chaperone effect in folding-unfolding dynamics of proteins under mechanical force, using magnetic tweezers.


 0 kommentar(er)
0 kommentar(er)
